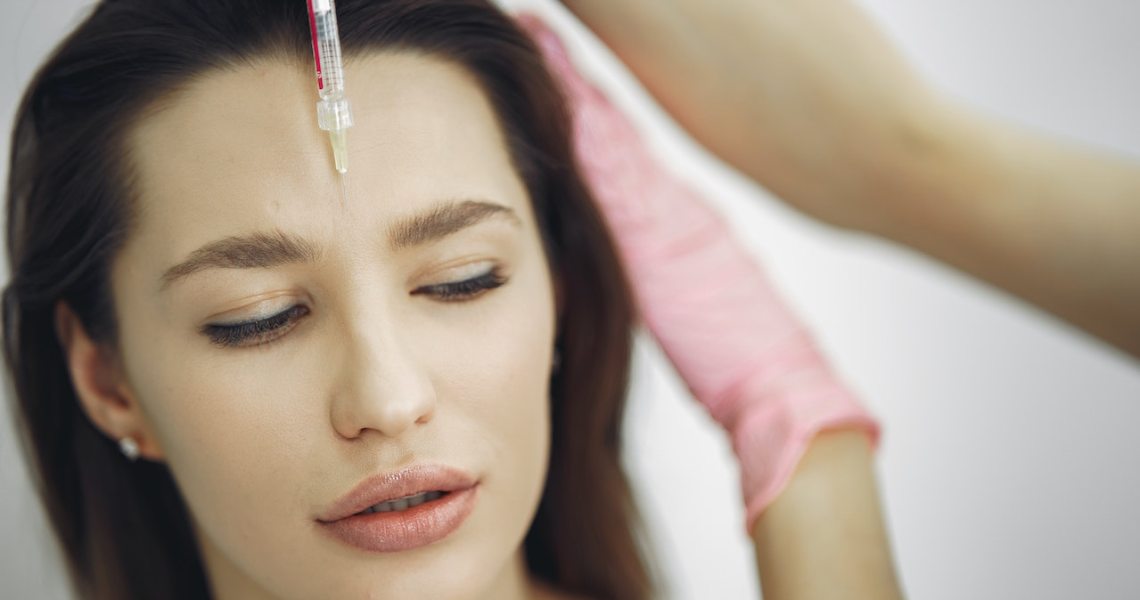
This week, I explore the latest development in the aesthetic injectables space, with the FDA approval of Allergan’s new hyaluronic acid intradermal microdroplet injection, Skinvive, and the impact it can have on the U.S. market.
A new aesthetic treatment has finally arrived on the U.S. market, ushering in a new class of injectables that could have a wide-sweeping impact.
In May, Allergan received FDA approval for Skinvive by Juvéderm, and in early October, it became available for patients to receive as a treatment. Skinvive is a hyaluronic acid-based treatment that promises to offer skin smoothness and a refreshed appearance by injecting hydration into the top layers of skin. The treatment lasts approximately six months.
Typically, traditional hyaluronic acid fillers like Juvéderm require injection just above bone to offer structure and plumping to the face and wrinkles. However, Skinvive does not function as a product to lift or add dimension but instead offers direct hydration and, therefore, luminosity and smoothness to the skin. And Botox, as a neuromodulator, is an entirely different class of wrinkle relaxers that inhibit muscle movement.
While the American audience has only begun learning about Skinvive and the similar skin-booster category, Europeans have been basking in the category’s glory for several years.
Skin boosters have been picking up steam in the U.S. over the past year due to media reporting on the category’s popularity in Europe. Skinvive has been available in Europe since 2017 and marketed under the name Volite. “Skin boosters” is a colloquial term referring to intradermal injectables, but also serums, treatments and even devices. For this reason, Allergan does not consider Skinvive a skin booster but instead a hyaluronic acid intradermal microdroplet injection. It’s the first and only intradermal microdroplet injection treatment approved for use in the U.S. According to Allergan, more than 47 million consumers are bothered by a skin quality issue, including dull skin lacking glow. And 85% of those people have considered addressing those skin quality issues with a treatment.
“With the launch of Skinvive by Juvéderm, the next class of injectables – skin quality treatments – is here, aimed at smoothing and hydrating instead of volumizing and lifting,” said Carrie Strom, president of Global Allergan Aesthetics and svp of parent company Abbvie. “We strongly feel that Skinvive has created an entirely new hyaluronic acid-based aesthetic treatment category that has the potential to expand the aesthetic injectables market.”
Ad position: web_incontent_pos1
Other skin booster injectables include Galderma-owned Restylane Vital, approved as a skin booster in Europe and available in the U.S. only for lips as Restylane Silk. There is also Revance-owned RHA Redensity, Merz-owned Belotero and IBSA-owned Profhilo. In the U.S., some of these products are used in an off-label capacity as “skin boosters” since they can be used as injectable skin hydration products but technically do not have FDA approval for that specific use case. Though the intended end result is an improved skin appearance based on smoothness and hydration, Skinvive is the first treatment specifically designed for this use and FDA-approved for it. From a strategic and advertising standpoint, manufacturers can only advertise approved indications, which means Allergan has the chance before any other competitor’s products to turn “Skinvive” into as big a household name as “Botox.”
Vanessa Coppola, nurse practitioner and founder of New Jersey-based aesthetic medical spa Bare Aesthetic, said she uses Skinvive in her practice, describing it as a “beautiful” product that “floods” the skin with water-loving hyaluronic acid and ultimately provides a refreshed and luminous appearance. Call it an injectable moisturizer, if you will.
“This is exciting in the aesthetics world and is just the tip of the iceberg,” said Coppola. “We’re going to see a lot of products coming [into the market] … focused on the appearance of surface-level skin. A few years ago, when everyone was talking about the K-beauty glass skin trend — this can offer that hydrated appearance.”
The publication Fashionista also recognized the impact Skinvive could have on the “high maintenance/low maintenance” beauty routine, wherein people receive high maintenance beauty routines like gel manicures, brow lamination and lash extensions to remain low maintenance in their day-to-day lives. In the case of Skinvive it would offer an ongoing hydrated and refreshed appearance, rivaling moisturizer. Meanwhile, Strom indicated that Skinvive could serve as a post-treatment top-off paired with lasers or microdermabrasion. Botox and other neuromodulators can already be partnered simultaneously with topical skin services like VI Peel (called ToxBooster treatment). Skinvive can serve as a skin recovery product, complementing or even competing with post-treatment-focused brands like Elta MD, SkinCeuticals, Skinbetter Science, Alastin and SkinMedica, which heavily distribute in the professional channel.
Coppola said that Skinvive is particularly exciting for post-menopausal women because hormonal changes during menopause can cause the skin to feel chronically dry. She added that Skinvive could be a great “top-off” to Sculptra, a bio-stimulating treatment by Galderma, because Sculptra helps rebuild a patient’s collagen. In her practice, she has seen a 150% increase in Sculptra services over the past year, while traditional fillers aside from lip augmentation, have declined. But feels that Skinvive could be a service that former-filler patients may turn to instead to reduce wrinkles. According to Precedence Research, the North American dermal fillers market size was estimated at $2.6 billion in 2022.
Ad position: web_incontent_pos2
“We see Skinvive as a product for Juvéderm-naïve patients who are interested in improving cheek skin smoothness and hydration because they have dry skin, as well as a treatment for seasoned injectable patients who are losing hyaluronic acid and have crepe-y skin due to aging,” said Strom.
Allergan’s fourth-quarter and full-year 2022 earnings, released in February, showed a 25.4% decline in the global net revenues for the five fillers comprising Juvéderm Collection, to $322 million. The company’s recent second-quarter 2023 earnings from July showed a modest increase, with global net revenues increasing 6.9% year-over-year to $368 million due to international growth. Juvéderm Collection sales still declined 14.5% in the U.S. year-over-year.
Glossy could not determine whether any of Allergan’s competitors are currently seeking FDA approval for their versions of intradermal skin boosters, but given the competitive nature of the aesthetic category, there could be several options on the market over the next few years. In the meantime, Allergan will deploy a multipronged marketing, public relations and influencer strategy at an undetermined time to educate consumers about the new injectable category and the benefits of Skinvive. The focus of the campaign will be how the treatment differs from traditional fillers that augment and add volume to the treatment area. Additionally, Allergan will have a “robust” injector marketing program to encourage practitioners to use and inform patients about Skinvive.
Inside our coverage:
Dove tackles stigma related to armpit insecurity.
Maybelline finds success with Reddit campaign.
Christian Louboutin released a more affordable lipstick.
What we’re reading:
How food changed the way we think of beauty.
JLo Beauty enters Macy’s.
Rare Beauty’s ascent to a Gen-Z behemoth.